Stopped Flow FAQs
What is stopped-flow technology?
- Typically used to gain an understanding of reaction mechanisms, including drug-binding processes or following protein structural changes, stopped-flow spectroscopy enables the study of fast reactions in solution over timescales in the range of 1 millisecond to hundreds of seconds.
What are the applications of stopped-flow?
- A wide range of reactions can be investigated involving, for example, protein-protein interactions, ligand binding, electron transfer, fluorescence resonance energy transfer (FRET), protein folding, and enzyme, chemical or coordination reactions.
How does the stopped-flow technology work?
- In most experimental setups, two reagents are rapidly mixed together and then ‘stopped’ in an observation cell. The sample cell is irradiated with monochromatic light. As the reaction proceeds, the change in the recorded signal, usually a fluorescence signal or absorbance at a specific wavelength, is recorded as a function of time.
Analysis of the resulting kinetic transient can determine reaction rates, the complexity of the reaction mechanism, information on short-lived reaction intermediates etc. A series of stopped-flow experiments can be used to show the effect of parameters such as temperature, pH and reagent concentration on the kinetics of a reaction.
What does a typical stopped-flow design look like?
- In an SX20 stopped-flow spectrometer, two optical pathlengths are available for absorbance detection (2 mm and 10 mm, or 1 mm and 5 mm, depending on cell type). The mixer is an integral part of the observation cell and has a dedicated observation window for fluorescence detection.
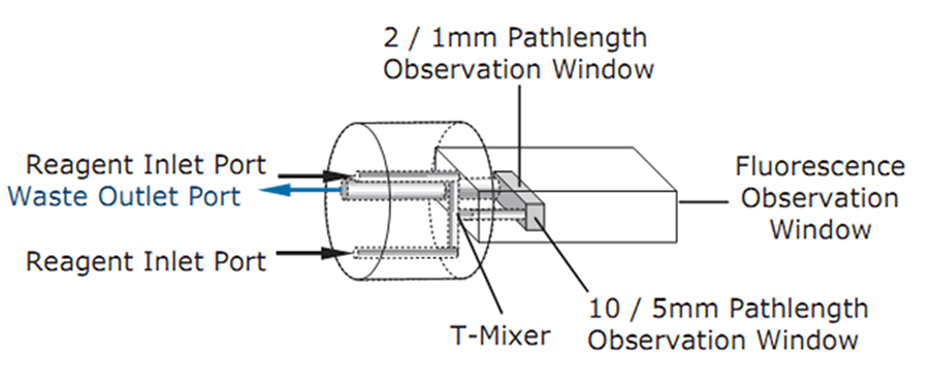
Schematic of the observation cell in an SX20 stopped-flow spectrometer
What is single mixing in stopped-flow technology?
- The stopping mechanism is a stop-syringe. Reagents are contained in two drive syringes (C and F). A drive ram pushes the syringe pistons such that the reagents are pushed through flow tubing to a mixer and then to the observation cell. This process pushes the ‘old’ cell contents towards the stop-syringe. The flow fills the stop-syringe, until the piston hits the trigger switch. This action simultaneously stops the flow and starts data acquisition. At this instant, the age of the reaction of the newly-mixed reagents in the observation cell is about one millisecond. The exact age (called the dead time) depends on the design of the stopped-flow instrument and the observation cell.
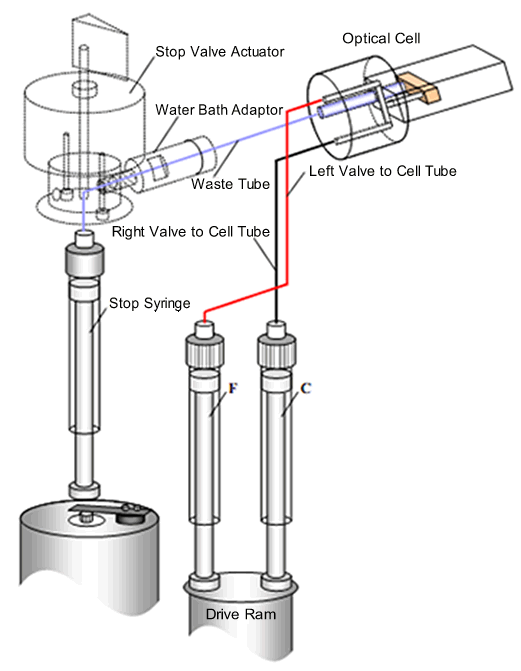
Schematic of an SX20 stopped-flow spectrometer - single mixing
What is sequential mixing in stopped-flow technology?
- Sequential mixing mode, also called double mixing, is a variation of the stopped-flow technique particularly well-suited to studying reactions between a short-lived reaction intermediate and a third reagent.
In a sequential mixing experiment, two reagents (A and B) are rapidly mixed and held in an ageing loop. After a pre-set interval (milliseconds to 10’s of seconds), the loop contents are rapidly mixed with a third reagent (C) in the observation cell, and the reaction (between the short-lived-intermediate and C) will be followed in the same manner as for the single mixing mode.
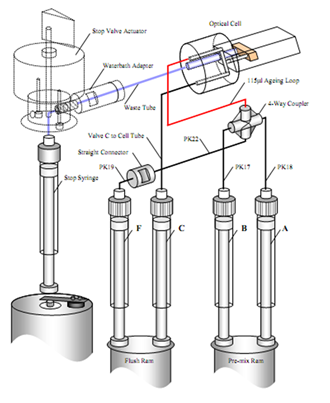
Schematic of an SX20 stopped-flow spectrometer - sequential mixing with option SX/SQ

