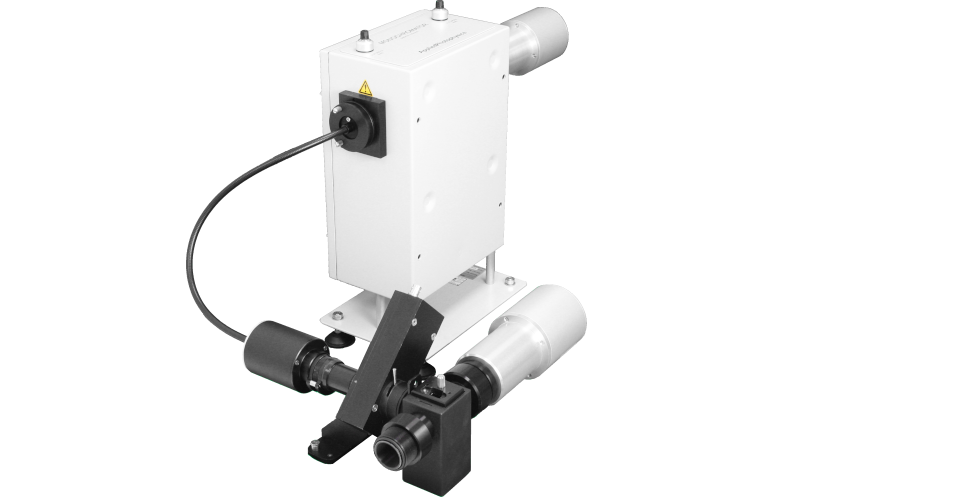
For high-quality fluorescence emission spectra
Chirascan
Scanning Emission Monochromator

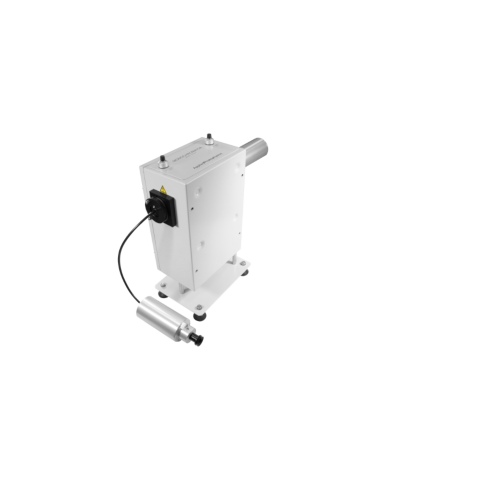
For measurements under limited absorbance conditions
Chirascan
Optical Rotary Dispersion
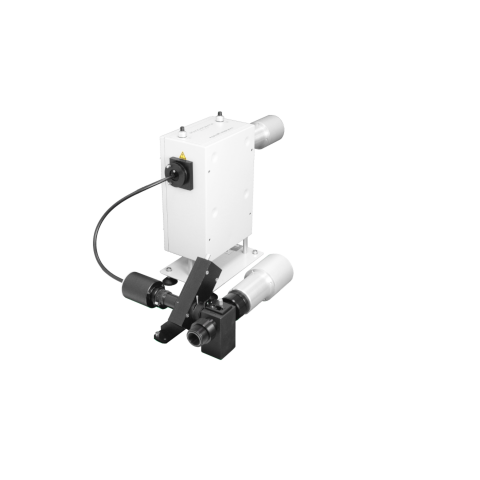
For information about the excited states of chiral molecules
Chirascan
Circularly Polarised Luminescence

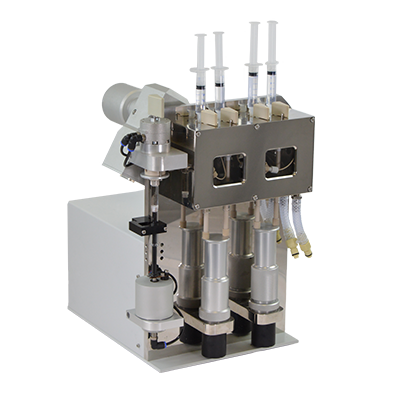
insight into protein-protein interactions, enzyme-substrate binding
SX20
Dual Fluorescence

easy-to-fit, dual channel, T-format fluorescence polarimeter
SX20
Fluorescence Polarisation